Hernia Mesh Failures and Injuries
Many patients have been hurt by defective surgical mesh used in hernia surgery
Building on our experience investigating and litigating claims involving defective transvaginal mesh and metal-on-metal artificial hip implants, SUGARMAN has formed a team to represent those who have been injured as a result of defective mesh products used in hernia surgery. This team is led by attorneys David McCormack and Stacey Pietrowicz.
Surgical mesh is meant to strengthen weakened areas of the body’s linings, and help new tissue grow. It has become widely used to treat hernias, which can occur when intestine or other internal organs push through a weakness in the abdominal muscles.
Unfortunately, these surgical mesh products are increasingly being recalled, withdrawn from the market, or investigated by the Federal Drug Administration (FDA) due to the serious and long-term health risks that the implanted mesh can cause.
This page provides information about hernias, hernia mesh injuries and failures, and your potential options for legal recourse if you have been hurt by these defective medical products.
About Hernia Mesh, Complications, and Failures
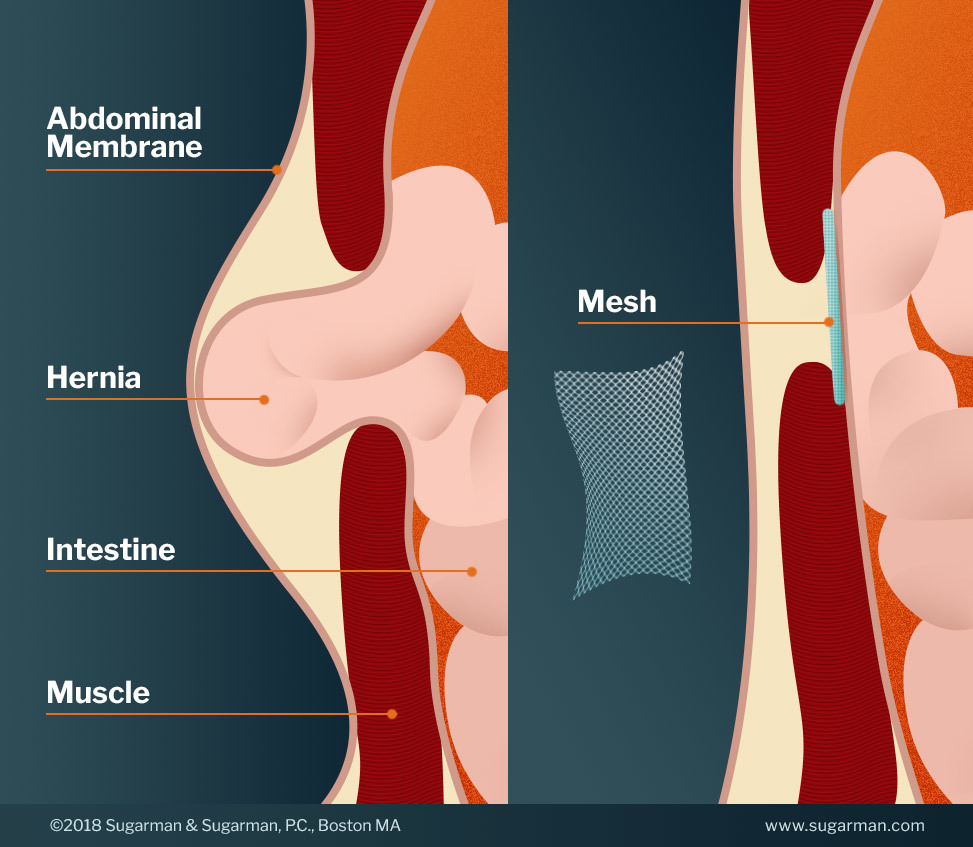
What is hernia mesh?
Surgical mesh is supposed to strengthen the weakened area and act as a catalyst for new tissue growth. It is made in both knitted and non-knitted sheets. Typically, it’s composed of synthetic materials or animal tissue. Mesh made from synthetic material can be absorbable, non-absorbable, or a combination. Mesh made from animal tissue (such as intestine or skin) are absorbable.
Non-absorbable mesh is considered to be a permanent implant, whereas absorbable mesh degrades over time and new tissue growth is supposed to aid in that repair.
What is the issue?
Surgical mesh is a medical device which has been used for more than three decades for hernia repair. Meshes are made from porous absorbable material, non-absorbable synthetic material, or absorbable biologic material. In most hernia mesh products, the manufacturer typically uses polypropylene, a synthetic plastic or polymer, to make the mesh; animal products are sometimes used as well.
These mesh products are known to stick to structures inside the body, shift from their original implant spot, curl, and become brittle, among other complications.
What kind of injuries does defective hernia mesh cause?
Common injuries include:
- Severe infections
- Adhesion of hernia mesh to organs
- Recurrence of hernias
- Pain and swelling
- Need for additional surgery to remove or revise the hernia mesh
- Bowel obstruction
How is the FDA involved (or not?)
Almost all defective hernia mesh products were approved by the FDA through the controversial 510(k) process. This allows manufacturers to bypass the full FDA testing process by showing their product is similar to an earlier product that did undergo full testing prior to approval. Most concerning, this loophole eliminates the need for a manufacturer to fully test and demonstrate to the FDA that its mesh product is safe for use in patients.
This has resulted in FDA-approved, but unsafe, medical devices (including transvaginal mesh, and Stryker and DePuy artificial hips) being implanted and causing severe and lifelong damage to patients.
On its website, the FDA discusses complications caused by hernia mesh surgeries, but provides little in the way of relevant patient safety information – the link on the page for recall notices directs patients to a notice involving counterfeit mesh products. The FDA has done little to educate patients regarding the risks of hernia mesh products and even less to take defective products off of the market.
Current Hernia Mesh Products that SUGARMAN is Investigating
Johnson & Johnson/Ethicon’s Physiomesh
In May of 2016, Johnson & Johnson, a subsidiary of Ethicon, announced that it was withdrawing its Physiomesh flexible composite mesh products, which was approved for sales in the U.S. through the 510(k) process in April of 2010. Johnson & Johnson took the withdrawal action after two unpublished scientific studies in Europe revealed that Physiomesh had a substantially higher revision rate than other hernia mesh products. This means that patients implanted with Physiomesh experienced more frequent recurrences of hernias and surgeries to repair the implanted hernia mesh. The FDA, however, did not formally issue a recall for these products and did not issue a Medical Device Safety Alert.
Despite the FDA’s inaction, its website contains dozens of Adverse Event Reports from physicians detailing the safety concerns and complications that they have experienced with patients who received a Physiomesh implant.
Based on the complications that this hernia mesh causes, a Multidistrict Litigation proceeding was established in the Northern District of Georgia. Patients from around the country bringing lawsuits as a result of defective Physiomesh products are now consolidated in that federal court district.
Atrium Medical Corp.’s C-QUR Hernia Mesh
Atrium is one manufacturer that the FDA has repeatedly warned about its hernia mesh products. In addition to warning letters relating to sanitary and sterilization concerns, the FDA has also received dozens of Adverse Event Reports from surgeons detailing the defective nature of this hernia mesh product and the harm that it causes to patients.
To date, the FDA has not issued a formal recall or Medical Device Safety Alert concerning the C-QUR hernia mesh. This does not, however, mean that the hernia product is safe for patients. Due to the number of patients experiencing severe injuries from C-QUR mesh, a Multidistrict Litigation proceeding has been created in the federal court for the District of New Hampshire with hundreds of cases filed from injured patients nationwide.
What are my options?
Patients obviously want to know what they should do if their hernia mesh has been recalled, if they were a Physiomesh or C-QUR mesh implant recipient, or if they are are experiencing any complications from their hernia mesh. First and foremost, call your doctor and have a frank discussion regarding what medical treatment, if any, is necessary to address your hernia mesh implant.
After consulting your doctors, give the personal injury lawyers at SUGARMAN a call so we can review your medical history and determine whether or not you are able to bring a claim under the law for any damages or injuries caused by the hernia mesh implanted in you.
First call your doctor. Then contact SUGARMAN.
What is a Hernia?
A hernia occurs when a part of an organ is displaced and bulges out through the wall of the surrounding muscle or connective tissue. Often, this occurs at a weak point in the abdominal wall near the intestines. Hernias are caused by a combination of pressure and an opening or weakness of muscle or connective tissue. This weakness can be present at birth but typically happens later in life. Anything that causes abdominal pressure can result in a hernia. Some examples include: obesity, lifting heavy objects, diarrhea or constipation, or persistent coughing or sneezing. Other contributing factors are nutrition, smoking, and overexertion.
What are symptoms of a hernia?
- A visible bulge on either side of your pubic bone. (It tends to become more obvious when you’re upright, particularly if you cough or strain.)
- A burning or aching sensation at the bulge.
- Pain or discomfort in your groin, especially when bending over, coughing or lifting.
- A heavy or “dragging” sensation in your groin.
- Weakness or pressure in your groin.
- Pain and swelling around the testicles when the protruding intestine falls into the scrotum.
Where do hernias happen?
- Inguinal: occurs in the inner groin
- Femoral: occurs in the upper thigh/outer groin
- Incisional: occurs through an incision or scar in the abdomen
- Ventral: occurs in the general abdominal/ventral wall
- Umbilical: occurs at the belly button
- Hiatal: occurs inside the abdomen, along the upper stomach/diaphragm
How serious are hernias?
If left untreated, a portion of your intestine could become trapped in your abdominal wall. This can obstruct your bowel and cause intense pain, nausea, and/or constipation. This is called an incarcerated hernia. When an incarcerated hernia cuts off blood flow to parts of your intestine, it is called a strangulation hernia. A strangulated hernia is life-threatening and requires immediate medical care.
